Now ACCepted
With the US Pharmacopeia’s approval of Chapter <86>, biopharmaceutical manufacturers can more easily incorporate (rCR) testing methods into their quality testing. Plus, PyroSmart NextGen® enhances your sustainability profile by eliminating the need for horseshoe crab blood. This synthetic alternative reagent reduces supply chain challenges while supporting your ESG goals. Synthetic LAL provides a sustainable, animal-free alternative to traditional LAL, offering high sensitivity, specificity, and stability.

Product Details
Sensitivity
The sensitivity for recombinant chromogenic assays is determined by the lowest standard concentration on the standard curve used for the assay. The maximum sensitivity of PyroSmart NextGen® is 0.005 EU/mL when run in an incubating microplate reader (or 0.001 EU/mL when run in Pyros Kinetix® Flex tube reader).
Sample to Lysate Ratio
PyroSmart NextGen® is used at economical volumes:
- Pyros Kinetix® Flex tube reader: 4:1 using 200 µL of test sample: 50 µL of reagent
- Microplate reader: 1:1 ratio using 50 µL of test sample: 50 µL of reagent
Performance
The PyroSmart NextGen® reaction mixture is incubated at 37±1°C and read in a microplate reader or tube reader equipped with a 405 nm filter. The incubation time depends on the lowest standard concentration in the standard curve, with 0.005 EU/mL achievable in 2,500 seconds in a microplate reader and 0.001 EU/mL achievable in 3,000 seconds in a tube reader, respectively. Software is used to construct the standard curve and calculate the endotoxin concentrations.
Stability
PyroSmart NextGen® is a lyophilized product with an excellent shelf life of 3 years from the date of manufacture.
Reconstitution
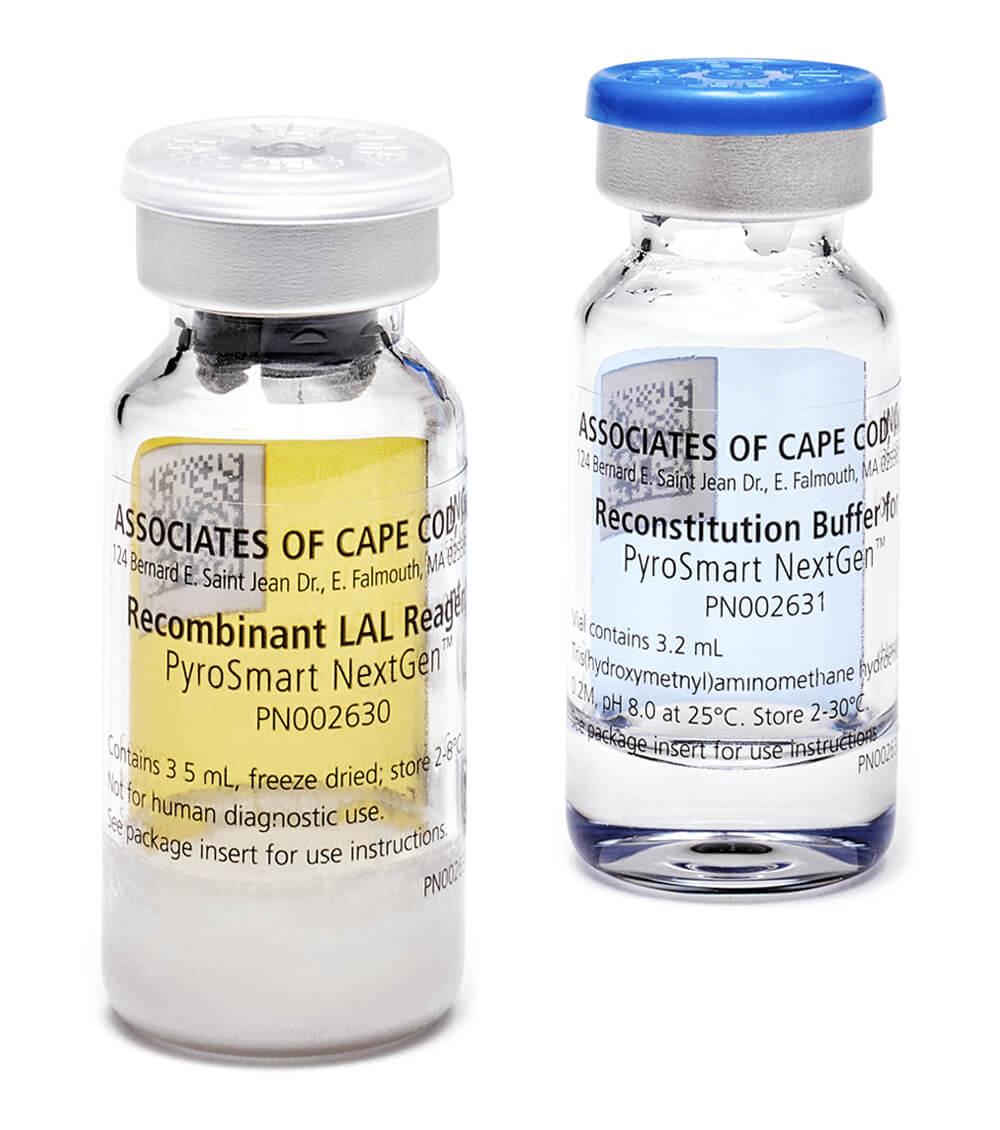
PyroSmart NextGen® is provided co-lyophilized with the chromogenic substrate, and as such, it is ready to use following a simple reconstitution (using 2.8mL of the supplied reconstitution buffer).
Product Packaging
PyroSmart NextGen® reagent is provided as a pack of 2 vials of reagent and 2 vials of reconstitution buffer. This is sufficient for a total of 96 wells (approx. 50 tests/vial).
Safety Data Sheets
Click on the links below to view Safety Data Sheets.
United States
PyroSmart NextGen® Recombinant LAL Reagent
PyroSmart NextGen® Reconstitution Buffer
United Kingdom
PyroSmart NextGen® Recombinant LAL Reagent (BE)
PyroSmart NextGen® Reconstitution Buffer (BE)
Europe
PyroSmart NextGen® Recombinant LAL Reagent (DE)
PyroSmart NextGen® Recombinant LAL Reagent (ES)
PyroSmart NextGen® Recombinant LAL Reagent (FR)
PyroSmart NextGen® Recombinant LAL Reagent (IT)
PyroSmart NextGen® Recombinant LAL Reagent (PT)
Pyrochrome® Reconstitution Buffer (DE)
Pyrochrome® Reconstitution Buffer (ES)
Pyrochrome® Reconstitution Buffer (FR)
Pyrochrome® Reconstitution Buffer (IT)
Pyrochrome® Reconstitution Buffer (PT)
Canada
PyroSmart NextGen® Recombinant LAL Reagent (FR)
Pyrochrome® Reconstitution Buffer (EN)
Pyrochrome® Reconstitution Buffer (FR)
Mexico
PyroSmart NextGen® Recombinant LAL Reagent (ES)
Pyrochrome® Reconstitution Buffer (ES)
Product Literature
Click on the links below to view product literature.
Package Inserts
PyroSmart NextGen® Product Brochure
BPS Reports
Vol. 6 No. 2 p.68-75 2023 Title: A Demonstration of the Validation Process for Alternative Endotoxin Testing Methods Using PyroSmart NextGen® Recombinant Cascade Reagent - Corresponding Author: Madeline Kelley
Vol. 6 No. 1 p.11-15 2023 Title: Evaluation of Recombinant Cascade Reagent PyroSmart NextGen® and Limulus Amebocyte Lysate Equivalency in a Plate and Tube Reader for Bacterial Endotoxins Testing - Corresponding Author: Madeline Kelley
Vol. 5 No. 5 p.105-114 2022 Title: Advanced Recombinant Cascade Reagent PyroSmart NextGen® for Bacterial Endotoxins Test as Described in the Pharmacopeias - Corresponding Author: Ingrid Stevens, Norihiko Ogura
Training Video
FAQs
How does PyroSmart NextGen® (PSNG) recombinant cascade reagent (rCR) work?
PSNG utilizes recombinant Limulus amebocyte lysate (LAL) reagent technology for bacterial endotoxin testing, designed to closely mimic the natural endotoxin detection cascade. This recombinant cascade reagent combines the essential components Factor C, Factor B, and pro-clotting enzyme, ensuring high sensitivity and accuracy in bacterial endotoxin testing while eliminating the potential for (1→3)-β-D-glucan cross-reactivity.
What are the advantages of PyroSmart NextGen® (PSNG) over kinetic and gel-clot BET methods?
PSNG recombinant cascade reagent offers a familiar workflow, using the same methodology, instrumentation, and software as traditional LAL reagents. With minimal changes to standard operating procedures and minimal technician training required, transitioning to PSNG is seamless, straightforward, and supported by ACC experts.
How is PyroSmart NextGen® (PSNG) more sustainable than traditional BET methods?
Unlike traditional BET methods, PSNG does not rely on horseshoe crab blood or any naturally sourced reagents. This recombinant cascade reagent is a sustainable solution that aligns with your environmental and ethical goals.
What types of endotoxin testing is PyroSmart NextGen® (PSNG) suitable for?
PSNG can be used for various endotoxin tests, ranging from standard water testing to samples requiring high sensitivity, such as intrathecal products, and those requiring high dilutions to overcome interference.
Will PSNG perform the same as naturally sourced reagents?
Yes, PSNG is a kinetic chromogenic reagent. It contains recombinant Factor C, Factor B, and pro-clotting enzyme. Thus, it continues to rely on the cascade mechanism to amplify the signal. It is co-lyophilized with the same chromogenic substrate as other chromogenic reagents. Therefore, it is homogeneous with Pyrochrome® and Chromo-LAL. This presents a considerable advantage to the end user: they keep the method and only switch out the reagent.
What’s the difference between Recombinant Factor C (rFC) and Recombinant Cascade Reagent (rCR)?
Recombinant Factor C reagent contains only recombinant Factor C. This enzyme acts as a biosensor for endotoxin (binds explicitly to it). However, due to the absence of the cascade amplifying mechanism (Factor B and pre-clotting enzyme), rFC reagents must be paired with a fluorescence method. This means that a fluorescent plate reader must be used (along with a specific microplate). This constitutes a different measured entity, different instrumentation, and different preparation steps with a limited output (endpoint assay only). Unlike PyroSmart NextGen®, which features single-step reconstitution, rFC reagents require three reagents in a specific ratio and a 10-minute pre-incubation step.
How are recombinant reagents produced?
Recombinant reagents are produced through a biopharmaceutical process based on the use of expression systems as cell line clones that contain (thanks to genetic engineering) DNA coding Factor C, Factor B and pro-clotting enzymes from Limulus polyphemus. The cell lines express the final glycoproteins Factor C, Factor B and pro-clotting enzyme, which are then collected, purified, and formulated into PyroSmart NextGen®.
Product Codes
| Product Description | Product Code |
|---|---|
| Multi-Test 2.8 mL/vial (approx. 50 tests/vial) | |
| Each kit contains: 2x PyroSmart NextGen® Reagent, 2x Reconstitution Buffer | #PNG050-2 |
Partner with the LAL Experts
Ready to adopt a sustainable LAL reagent technology for bacterial endotoxin testing? Contact us now to get expert guidance from the BET specialists at ACC. With our industry-leading support, we can help you transition from your current method to PyroSmart NextGen® recombinant cascade reagent. Our experienced team is here to support you every step of the way.
Contact us today to transform your BET journey.