LAL Reagents
For Bacterial Endotoxin Testing
High-Quality Products From the Company That Invented LAL Testing
Limulus amebocyte lysate (LAL) reagents, the gold standard for bacterial endotoxin testing, are known for their accuracy, helping to protect patient safety. ACC pioneered the LAL test and has been a leader in the industry for decades. In pharmaceutical manufacturing and renal dialysis centers, water testing, and other applications, customers rely on ACC’s LAL reagents and unparalleled customer support to provide a high level of confidence in accurate endotoxin detection.
The LAL endotoxin test is critical to ensuring product safety and compliance with regulatory standards. Our reagents for the LAL test procedure are backed by expert support and have a long-standing reputation for reliability. These endotoxin test methods are instrumental in maintaining patient safety and compliance with stringent regulatory standards, including those set by the United States Pharmacopeia (USP) and the European Pharmacopeia (EP).
Pyrotell®
Gel-Clot Endotoxin Test Reagent
Pyrotell® lysate was the first LAL gel-clot reagent licensed by the US FDA. It is easy to use and available in economical multi-test vials and convenient single-test vials (STVs). Pyrotell lysate is a robust reagent that produces firm, easily read...
Pyrotell®-T
Turbidimetric LAL Reagent
Pyrotell®-T lysate is used to quantify endotoxin using the kinetic turbidimetric method. This LAL reagent can be used for various endotoxin tests, ranging from standard water testing to samples requiring high sensitivity, such as intrathecal...
Pyrochrome®
Chromogenic LAL Reagent
Pyrochrome® is a versatile chromogenic LAL reagent designed for quantitative endotoxin testing in compliance with USP, EP, and JP bacterial endotoxin test chapters. This reagent is ideal for both kinetic and endpoint assays, offering high...
Chromo-LAL
Kinetic Chromogenic Reagent
Chromo-LAL is a high-performance kinetic chromogenic LAL reagent designed for precise endotoxin detection using microplate readers. This lysate is lyophilized with substrate reagents and buffers, providing a stable and robust solution optimized for...
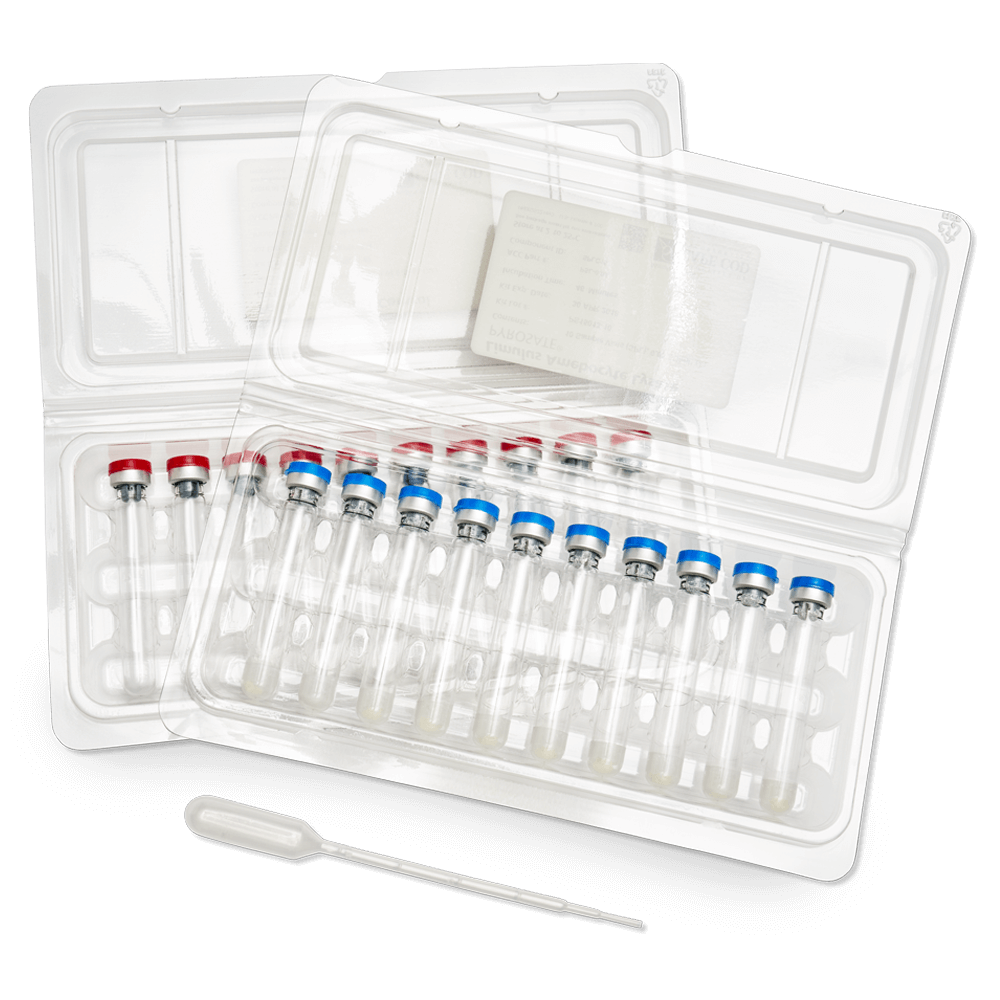
Pyrosate® Kit
Rapid Endotoxin Test Kit
The Pyrosate Kit is an accessible, straightforward, and rapid Limulus amebocyte lysate (LAL) test kit. No special training or laboratory supplies are necessary to perform the test, and the step-by-step illustrated instructions allow the user to...
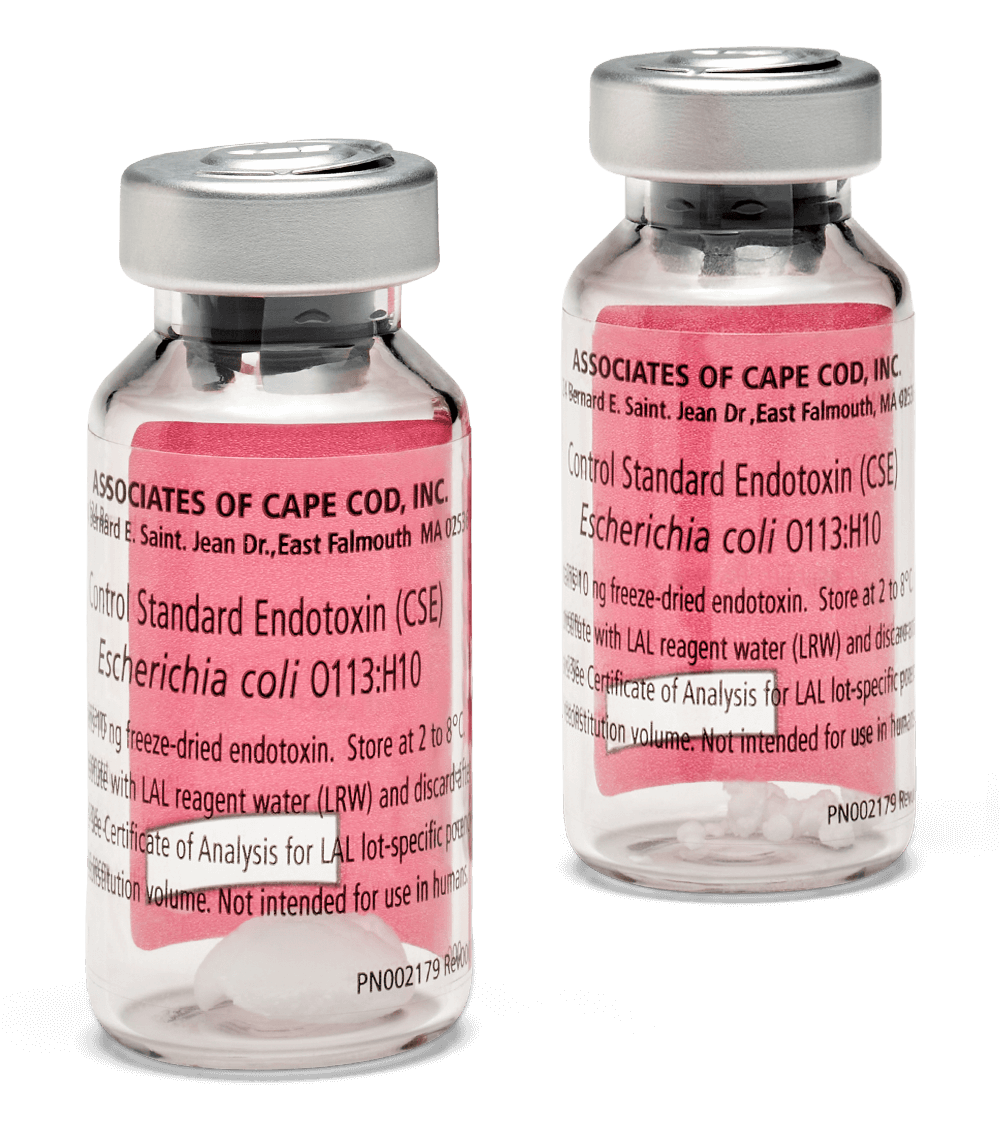
Control Standard Endotoxin
A Standard for Endotoxin Testing
Control Standard Endotoxin (CSE) is a widely used standard for endotoxin testing. It is a purified extract of Escherichia coli O113:H10, the same strain used for the United States Pharmacopeia and the European Pharmacopeia reference standard...
FAQs
What is endotoxin testing, and why is it important?
Bacterial endotoxin testing (BET) is vital for detecting the presence of endotoxins of gram-negative bacteria. These endotoxins can cause severe immune reactions in humans, making their detection essential in the production of injectable pharmaceuticals, medical devices, and other biological products. The LAL assay for endotoxin is the industry-standard method for endotoxin detection, ensuring the safety and efficacy of products before they reach the market.
What is Limulus Amebocyte Lysate (LAL)?
Limulus amebocyte lysate (LAL) is an aqueous extract of blood cells (amoebocytes) from the horseshoe crab.
What is the Limulus Amebocyte Lysate (LAL) endotoxin test, and how does it work?
The LAL test procedure uses horseshoe crab blood, which clots in the presence of bacterial endotoxins. This test is highly sensitive and can detect even minute amounts of endotoxins, making it a reliable method for ensuring product safety. Different types of LAL assays exist, including gel-clot, turbidimetric, and chromogenic methods, each designed to meet specific testing needs.
How do LAL reagents contribute to BET?
LAL reagents are the key components used in BET. These reagents react with endotoxins to produce a measurable response, whether it be clot formation, turbidity, or a color change, depending on the assay type. Reliable LAL reagents are essential for accurate and consistent test results, helping manufacturers comply with regulatory standards and protect patient safety.
What are the advantages of using LAL assays for endotoxin tests?
LAL assays are the most widely accepted method for endotoxin testing due to their sensitivity, reliability, and rapid detection of endotoxins. These assays are versatile, with different formats available to suit various testing environments, from simple gel-clot tests to more sophisticated kinetic assays. Every type of LAL assay helps manufacturers bring safe products to market faster, supporting the advancement of healthcare.
Can LAL testing methods be used for applications beyond pharmaceuticals?
Yes, LAL testing methods are used in various industries beyond pharmaceuticals, including medical devices, dialysis centers, and water testing. The ability to detect bacterial endotoxins in various samples makes the LAL assay essential for ensuring safety across different applications.

