Hardware + Software
Bacterial Endotoxin Testing Equipment
Discover ACC’s Industry-Leading Hardware and Software Solutions for a Complete Endotoxin Testing System
When performing bacterial endotoxin testing, laboratories face numerous challenges, including ensuring accuracy, maintaining compliance with global regulatory agencies, and managing the complexities of their testing environments. With the critical need to detect bacterial endotoxins in pharmaceutical and medical products, reliable and efficient endotoxin testing equipment, including instrumentation and software, is essential for safeguarding patient safety and product integrity. Overly complex or outdated systems can lead to inefficiencies, inaccurate results, or costly delays in product release.
One common challenge is integrating an endotoxin testing system into a cohesive workflow. Laboratories require sensitive and accurate instruments that are easy to use and compliant with regulatory requirements like 21 CFR Part 11. Endotoxin testing software eases integration by providing quantitative testing, data management, and reporting capabilities. When testing processes are cumbersome or inconsistent, they increase the likelihood of human error, incomplete data, or out-of-specification (OOS) results, which can be costly in terms of time and resources. Moreover, managing data security and traceability is critical in avoiding compliance issues, which can further complicate testing procedures.
For accurate and efficient endotoxin detection and analysis, ACC offers a comprehensive range of endotoxin testing equipment and systems designed to support the industry’s most demanding needs. Our advanced instruments are engineered to deliver precise and reliable results, while our cutting-edge endotoxin testing software ensures data integrity and compliance with regulatory standards. Learn how our easy-to-use systems can integrate seamlessly with your endotoxin testing operations.
Endotoxin Testing Hardware
ACC is proud to provide superior endotoxin testing equipment, including tube readers and a microplate reader to support all your testing needs.

Pyros Kinetix® Flex
Incubating Kinetic Tube Reader
Designed with flexibility and efficiency in mind, Pyros Kinetix® Flex Incubating Kinetic Tube Reader is designed for both chromogenic and turbidimetric endotoxin testing and provides the industry’s highest kinetic turbidimetric assay...

Agilent BioTek Epoch 2
Microplate Spectrophotometer
Efficient and accurate bacterial endotoxin testing is essential for ensuring the safety and efficacy of pharmaceutical and medical products. A versatile microplate reader is a key endotoxin testing system component. However, selecting the right...

Extended Warranty
For the Pyros Kinetix® Flex Tube Reader
An optional extended warranty covers our Pyros Kinetix® tube readers for up to seven years. With an extended warranty, laboratories using the Pyros Kinetix® tube reader can further protect their investment and ensure their equipment’s...
Endotoxin Testing Software
Reliable, precise, and easy-to-interpret data is essential to any effective kinetic method of endotoxin detection. Endotoxin testing and analysis software ensures data accuracy and compliance with regulatory guidelines.
ACC provides advanced software solutions to streamline your testing operations. These systems are all designed with efficiency and regulatory compliance in mind and feature 21 CFR Part 11 compliance, dynamic reporting options, and increased data security.
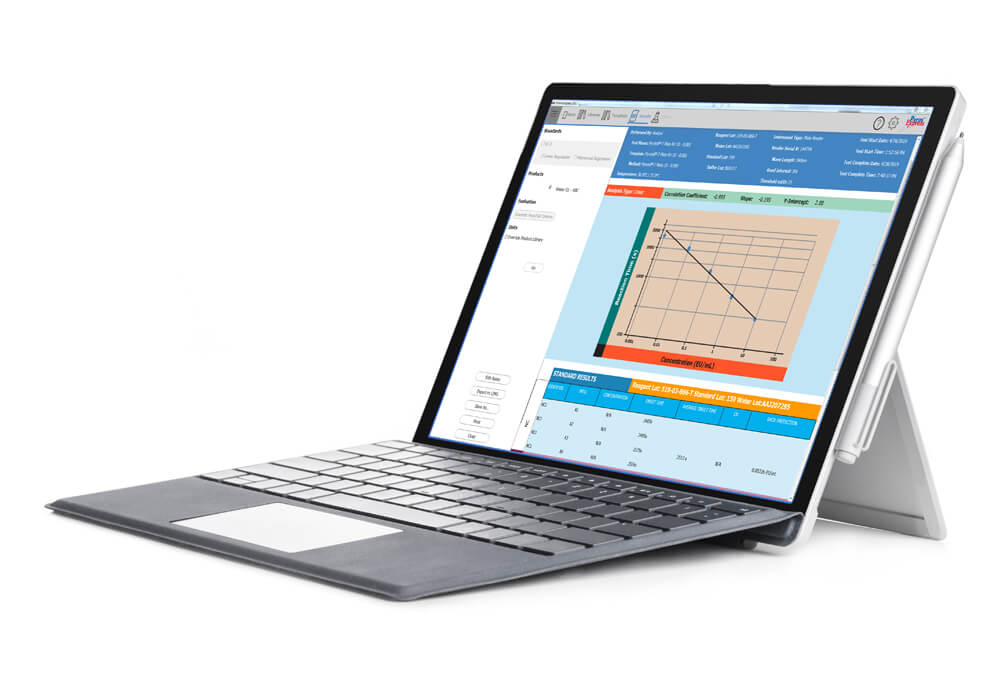
Pyros® eXpress
Endotoxin and Glucan Testing Software
Pyros® eXpress, ACC’s leading endotoxin and glucan detection analysis software, streamlines data collection and analysis for endotoxin and glucan testing, integrating seamlessly with ACC’s full range of testing systems. Designed to enhance...

Validation Protocols
Guided System Validation for Pyros® eXpress Software
The Pyros® eXpress Validation Protocols provide the end user with a comprehensive set of integrated documents to guide them through the system validation process.

Gen5® Secure Software
For the BioTek Epoch 2 Microplate Spectrophotometer
Gen5® Secure software was created for microplate users by engineers focused on microplate instrument technology. It offers a fully compatible, customized and 21 CFR Part 11 compliant microplate software program for use with the Agilent-BioTek Epoch...

